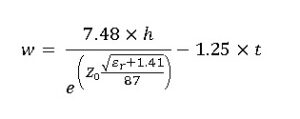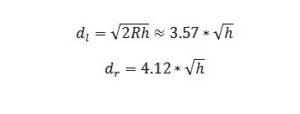Light exhibits properties of both particles (photons) and waves, a phenomenon known as wave-particle duality. Despite being considered as particles, photons also exhibit wave-like behaviors, including interference and diffraction. The frequency of light arises from its wave nature. In the wave model, light is an electromagnetic wave consisting of oscillating electric and magnetic fields traveling through space. The frequency of light corresponds to the number of oscillations per second of these electric and magnetic fields. This frequency determines the color of light and is directly related to its energy through Planck’s relation E=hνE = h \nuE=hν, where EEE is the energy of the photon, hhh is Planck’s constant, and ν\nuν is the frequency of light.
Light particles, or photons, do not have a frequency in the traditional sense that waves do. Instead, photons are quanta of electromagnetic radiation with a specific energy and wavelength associated with their frequency. The frequency of light is a property of the electromagnetic wave that photons collectively form when traveling through space. Each photon carries energy proportional to its frequency, and the cumulative effect of many photons determines the intensity and properties of the light wave observed.
The dual nature of light as both a particle and a wave arises from experimental observations and theoretical developments in physics, particularly in the early 20th century with the advent of quantum mechanics. In some experiments, light exhibits behaviors that are characteristic of particles, such as the photoelectric effect, where photons eject electrons from metals. In other experiments, light displays wave-like phenomena, such as interference patterns observed in Young’s double-slit experiment. The wave-particle duality of light and other quantum entities challenges classical concepts of particles and waves, suggesting that these entities exhibit properties of both depending on the experimental setup and observation.
The frequency of light remains constant because it is a fundamental property of the electromagnetic wave that light forms. In vacuum, the speed of light ccc is constant, approximately 3×1083 \times 10^83×108 meters per second. The frequency ν\nuν and wavelength λ\lambdaλ of light are related by the equation c=λνc = \lambda \nuc=λν, indicating that as the wavelength changes, the frequency must change inversely to maintain this relationship. In different media, such as air or glass, the speed of light may vary, but within each medium, the frequency of light remains constant because it is an intrinsic property of the electromagnetic radiation emitted or absorbed by atoms or molecules. Thus, regardless of the medium through which light travels, its frequency remains a characteristic feature that determines its color and energy.