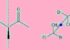
Diastereomers (sometimes called diastereoisomers) are a type of stereoisomer. Diastereomerism occurs when two or more stereoisomers of a compound have different configurations on one or more (but not all) of equivalent (related) stereocenters and are not mirror images of each other.
Diastereomers (sometimes called diastereoisomers) are a type of stereoisomer. The sereereomerism occurs when two or more stereoisomers of a compound have different configurations in one or more (but not all) of equivalent (related) stereocenters and are not mirror images of each other. When two diastereoisomers differ from one another to a single stereocentre they are epimers. Each stereocentre gives rise to two different configurations and therefore increases the number of stereoisomers by a factor of two.
Diastereomers differ from enantiomers because they are pairs of stereoisomers that differ in all stereocenters and are therefore mirror images of each other. The enantiomers of a compound with more than one stereocentre are also diastereomers of the other stereoisomers of that compound which are not their mirror image.
Diastereomers have different physical properties (unlike enantiomers) and different chemical reactivity. Diastereoselectivity is the preference for the formation of one or more diastereomers on the other in an organic reaction.