The terms constitutional isomers and stereoisomers form two broad categories of isomers (molecules of the same chemical formula). Constitutional isomers usually have different connectivity, and stereoisomers have the same connectivity, but differ in spatial arrangements.
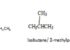
Structural isomers have the same molecular formula, but a different binding arrangement among atoms. The stereoisomers have identical molecular formulas and atomic arrangements. They differ from each other only in the spatial orientation of the groups in the molecule.
Isomeric isomers are stereoisomers that can not be converted to each other by rotating the molecule around a single bond. These configurational isomers are found in two types as geometric isomers and optical isomers.
Geometric isomers are also called cis-trans isomers. This type of isomerism is found mainly in Alkanes and rarely in alkanes.
Geometric isomerism describes the presence of two identical groups (which are attached to the vinyl carbon atoms) positioned on the same or opposite side of the double bond. If the two identical groups are on the same side, it is called the cis-isomer and if the two identical groups are on opposite sides, it is called the trans isomer.